/reboot/media/e2ff1b32-8f47-11e8-b37b-fa163e14ea56/06444f34-3200-11ea-b800-0242ac130005/0-0-t8sel-r-005.jpg)
Pulsed light disinfection: why do we have to keep the germs in sterile water?
The impact of germs maintained in salt for pulsed light treatment
Introduction
The VDMA [1] specifies in its practice code, which allows to test the efficiency of the sterilisation devices on packaging, that the inoculums should be prepared without salt. Indeed during the drying of the inoculums the presence of salt forms like a protective layer on it and it has an important impact on the decontamination results.
Just a few scientific papers on pulsed light sterilisation report about this factor. Some of them report about the impact of salt and fat on the pulsed light decontamination without specifying that for the salt the impact is only when the inoculum is already dry.
The salt concentration of the inoculation liquid is therefore a considerable factor to take in consideration.
Equipment and method
In order to highlight the impact of salt on the decontamination, we conducted a series of tests.
For this experiment we choose to use ascospores of Aspergillus brasiliensis DSM1988 (reference germ for UV decontamination of surfaces).
The inoculum was prepared to obtain 3 logs per bioindicator (BI) at different salt concentrations.
We decided to test 0, 4, 6 and 8.5 g/l of salt. The concentration of 8.5 is the Tryptone salt containing salt, the usual laboratory diluent.
The BIs were processed with the STERIXENE XS150 driver at 1.4 J / cm² fluence.
Results
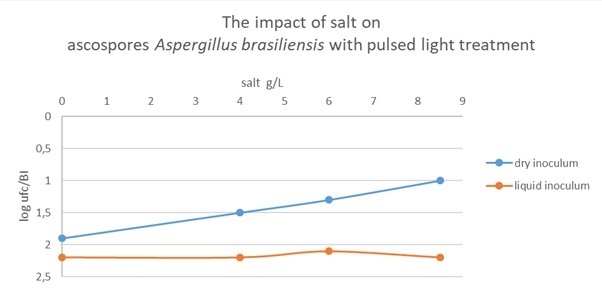
When the sample is in a liquid form, the salt concentration has no impact on the potential destruction by pulsed light.
When the sample is in a dry form, the presence of salt at 8.5g/l decreases of 1 log the potential destruction by pulsed light compared with the salt‑free tests.

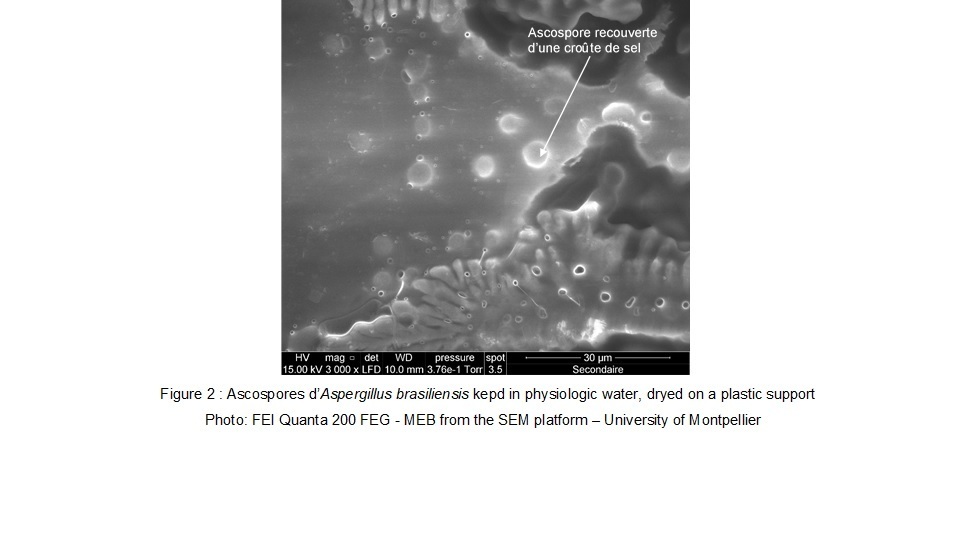
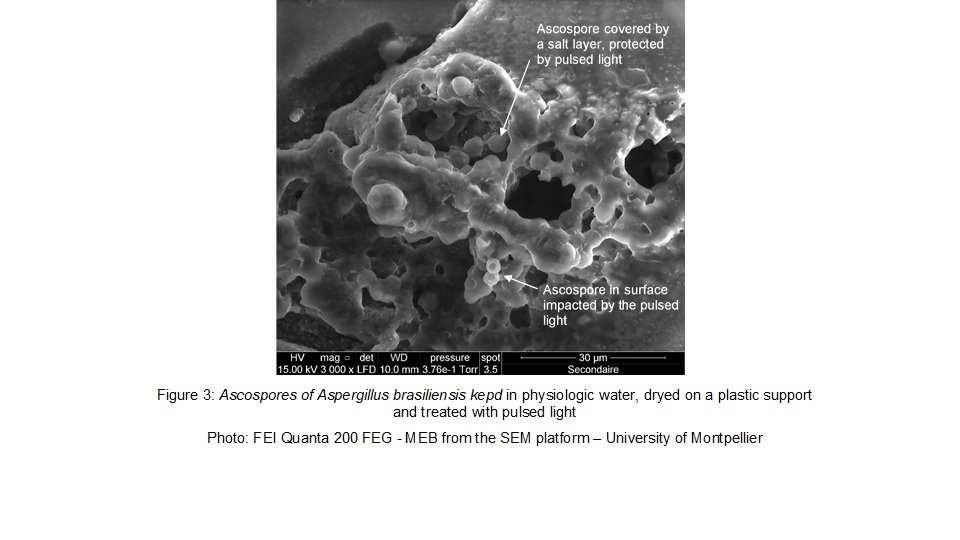
On the electronic microscope images we can observe, that the Aspergillus brasiliensis ascopores are protected by a salt layer. We can observe on the second figure, that the surface ascopores had an impact by the pulsed light treatment.
Conclusion
The presence of salt crystals on the surface of a bioindicator can have a significant impact on the performance of the pulsed light treatment
This is the reason why it is very important, for the verification of pulsed light and UV sterilization devices, to make sure, that the bioindicators, or spot/spray inoculation on surface have been done with salt‑free inoculum.
Acknowledgement:
The autor would like to mention Frédéric Fernandez for the MEB images from the SEM university of Montpellier.
https://mea.edu.umontpellier.fr/
References’:
[1] VDMA, 2008. Code of Practice Filling Machines of VDMA Hygiene Class V: Testing the Effectiveness of Packaging Sterilization Devices - https://www.vdma.org/en/
[2] LEVY, C. (2010). Principaux facteurs influençant l’efficacité de la lumière pulsée pour la décontamination des microorganismes pathogènes et d’altération des denrées alimentaires. https://tel.archives-ouvertes.fr/tel-00747302/document
:strip_exif()/reboot/media/e2ff1b32-8f47-11e8-b37b-fa163e14ea56/c670c6e8-2d4b-11ef-a101-0242ac120012/1-1-logo-sterixene-pulsed-light-et-uv-led-decontamination-par-lumiere-pulsee-desinfection-sterilisation.png)
:strip_exif()/reboot/media/e2ff1b32-8f47-11e8-b37b-fa163e14ea56/c670c6e8-2d4b-11ef-a101-0242ac120012/1-1-logo-sterixene-pulsed-light-et-uv-led-decontamination-par-lumiere-pulsee-desinfection-sterilisation.png)

:recolor():strip_exif()/reboot/media/e2ff1b32-8f47-11e8-b37b-fa163e14ea56/a187805a-1807-11ea-a688-0242ac130003/0-0-microbiological-tests.jpg)
:recolor():strip_exif()/reboot/media/e2ff1b32-8f47-11e8-b37b-fa163e14ea56/4415dfee-4f38-11ea-90ac-0242ac130005/0-0-interieur-machine-3.jpg)
:recolor():strip_exif()/reboot/media/e2ff1b32-8f47-11e8-b37b-fa163e14ea56/79a440d2-1807-11ea-b135-0242ac130003/0-0-biological-indicators.jpg)